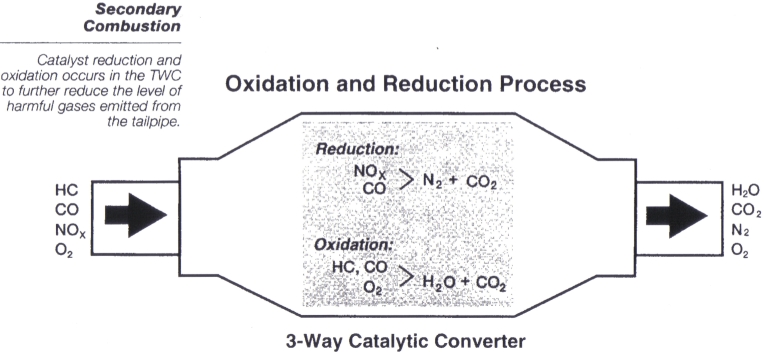
The three- way converter has three jobs the third job being the reason as to why two-way converters are now obsolete. C x H 2x2 3x12 O 2 x CO 2 x1 H 2 O a combustion reaction.
Newer high-end vehicles are now coming equipped with two catalytic converters as part of an even more stringent emissions program.
Two way catalytic converter. A 2-way or oxidation sometimes called an oxi-cat catalytic converter has two simultaneous tasks. Oxidation of carbon monoxide to carbon dioxide. 2 CO O 2 2 CO 2 Oxidation of hydrocarbons unburnt and partially burned fuel to carbon dioxide and water.
C x H 2x2 3x12 O 2 x CO 2 x1 H 2 O a combustion reaction. Allows oxidation of CO carbon monoxide to less-harmful CO2 carbon dioxide Allows oxidation of HC unburned hydrocarbons to CO2 carbon dioxide and H2O water In this design exhaust gases are directed to flow through the substrate containing precious metals platinum and palladium which allow the chemical reaction to occur. The two-way catalytic converter was present on vehicles in the United States until 1981.
They only have oxidation catalysts which help change carbon monoxide to carbon dioxide. Hydrocarbons which is unburned and partially burned fuel are changed to carbon dioxide and water. A two-way or oxidation catalytic converter has two simultaneous tasks.
Oxidation of carbon monoxide to carbon dioxide. 2CO O2 2CO2. Oxidation of hydrocarbons unburnt and partially burnt fuel to carbon dioxide and water.
CxH2x2 3x12 O2 xCO2 x1 H2O a combustion reaction This type of catalytic converter is widely used on diesel engines to reduce hydrocarbon and carbon monoxide emissions. A two-way catalytic converter is designed to do only two main things. One job is to oxidize the carbon monoxide to turn it into carbon dioxide and the other job is to oxidize the hydrocarbons or the unusedunburnt fuel and turn it into carbon dioxide and water.
The three- way converter has three jobs the third job being the reason as to why two-way converters are now obsolete. Newer high-end vehicles are now coming equipped with two catalytic converters as part of an even more stringent emissions program. The first converter breaks down the exhaust as normal while the second acts both as a filter for tiny particles and a pump which releases a chemical mixture that further reduces any harmful gases which may have escaped the first converter.
Some less-effective converters carry out only the second two oxidation addition reactions so theyre called two-way catalytic converters. After the catalyst has done its job what emerge from the exhaust is mostly oxygen nitrogen carbon dioxide and water in vapor form. Several men were arrested within 24 hours of each other for two catalytic converter thefts off cars in San Mateo and Foster City according to police.
A two way catalytic converter focuses on converting carbon monoxide to carbon dioxide and hydrocarbons to water. The function of a 3 way catalytic converter goes one step further by also converting nitrogen oxide to nitrogen. Catalytic converter theft comes in waves affecting various types of vehicles at random.
Unlucky victims will start their engines and hear a loud motorcycle-like vroom from their cars. Or you may have heard coworkers or neighbors complain nearly as loudly about experiencing a stolen catalytic converter themselves. The two-way catalytic converter is usually used in diesel engines.
It performs two tasks at the same time. Converts carbon monoxide into carbon dioxide. Changes hydrocarbon into carbon dioxide and water.
As a result of catalytic reaction the exhaust gases pass over the converter substance the toxic gases such CO HC and NOx are converted into harmless CO2 H2 and N2. Two way catalytic converter. Through catalytic action a chemical changes converts carbon monoxide CO and hydrocarbon HC into carbon dioxide CO2 and water oxidation.
Direct-fit catalytic converters are of three basic types. Two-way three-way and three-way plus oxidation converters. Two-way oxidation converters are used up to 1980 and are designed to eliminate hydrocarbons HC and carbon monoxide CO.
Three-way converters are designed to eliminate nitrogen oxides NOX as well. Catalytic Converter - Deconstructed. The illustration above shows the catalytic converters major components.
The main components are two honeycomb-like monoliths covered with a thin layer of PtRh first monolith and PdRh second. They provide the surface area where the reaction occurs. The only way to truly know the value of a load of scrap catalytic converters is through assaying.
Decanning and milling the ceramic subtract then collecting a sample will reveal how much each precious metal the lot contains. Assaying eliminates the one-by-one process which is time-consuming which in turn increase the return rate of the load. A Two-Way converter used on American cars between 1975 - 1980 oxidizes unburned harmful hydrocarbons and carbon monoxide into water and carbon dioxide.
The first vehicles with catalytic. Catalytic converters contain honeycomb-like structures coated with platinum and palladium that help minimize the harmful effects of carbon monoxide hydrocarbons and nitrogen oxide produced by your vehicles engine. These harmful elements pass through two stages.
The reduction catalyst stage and the oxidation catalyst stage. There are two types of converters in usethe two-way and the three-way units. The two-way oxidation catalyst was first added to new cars in model year 1975.
These units use platinum and palladium to oxidize the HC and CO and can meet the 041- and 34-gmile standards specified for these constitutents for 1981.