Stable pressure is equally important in your everyday car. Specialty Tires for Planes Race Cars and Heavy Equipment.
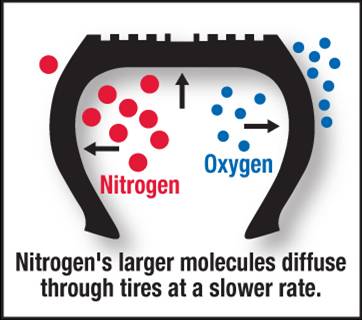
Nitrogen particles are larger than oxygen and this makes it more difficult for nitrogen to leak out of your tires.
Oxygen vs nitrogen in tires. Whats the Difference Benefits of Nitrogen Tires The rubber compounds in tires have microscopic pores through which eventually air and nitrogen will seep. Oxygen as Gas in Your New Tires Tires are designed to work best when they have the ideal inflation pressure inside. In fact its actually 21 percent oxygen 78 percent nitrogen and 1 percent other gases.
Air is 78 percent nitrogen and just under 21 percent oxygen and the rest is water vapor CO2 and small concentrations of noble gases. Because nitrogen is more stable than oxygen it is common in the tires of vehicles that require more precision tuning such as race cars industrial machinery aircraft and spacecraft. Basically when you pay extra for your nitrogen filled tires you are actually paying absence of the oxygen from the air the whole 21 of it.
The NHTSA study also found that regular air escapes tires at a higher rate than pure nitrogen. The government agency concluded that using nitrogen does reduce tire degradation by limiting. When filling a tire with compressed air its important to understand that its not just oxygen filling the tire.
Instead compressed air is made up of many other gases including a lot of nitrogen. In this video I talk about putting nitrogen vs air in your car tires. There are pros and cons to each of them and I outline them in the video.
Tires naturally lose small amounts of pressure over time whether they are filled with compressed air oxygen or nitrogen. Nitrogen is an inert non-flammable gas nothing more than dry air with the oxygen removed. Ambient air contains usually contains 78 nitrogen 21 oxygen and 1 miscellaneous gas.
Because of nitrogens inert properties it is often used in highly specialized tire service applications andor demanding environments. These applications usually include aircraft mining and commercialheavy use. Long-term tire aging and wheel corrosion.
Advantage for nitrogen Air is 78 nitrogen and 21 oxygen with the remainder being trace gases. Oxygen can retain moisture inside your tires and eventually can oxidize the internal tire wall casing causing premature tire aging. Nitrogen is an inert non-flammable gas basically nothing more than dry air with oxygen removed.
In fact ambient air contains about 78 nitrogen 21 oxygen and 1 miscellaneous gas. Because of nitrogens inert properties it is often used in highly specialized tire service applications andor demanding environments. The air in your tire is typically 78 percent nitrogen and 21 percent oxygen.
Due to this lower percentage of nitrogen concentration oxygen will retain moisture inside the tire. The moisture causes oxidation on the tire wall casing which can cause premature tire aging. Specialty Tires for Planes Race Cars and Heavy Equipment.
Air the standard gas used to fill car tires is comprised of 78 percent nitrogen. The rest of the gas is made of approximately 21 percent oxygen water vapor carbon dioxide and smaller concentrations of miscellaneous noble gases such as neon and argon. This combination of gases affects how the air in your tire behaves at different temperatures.
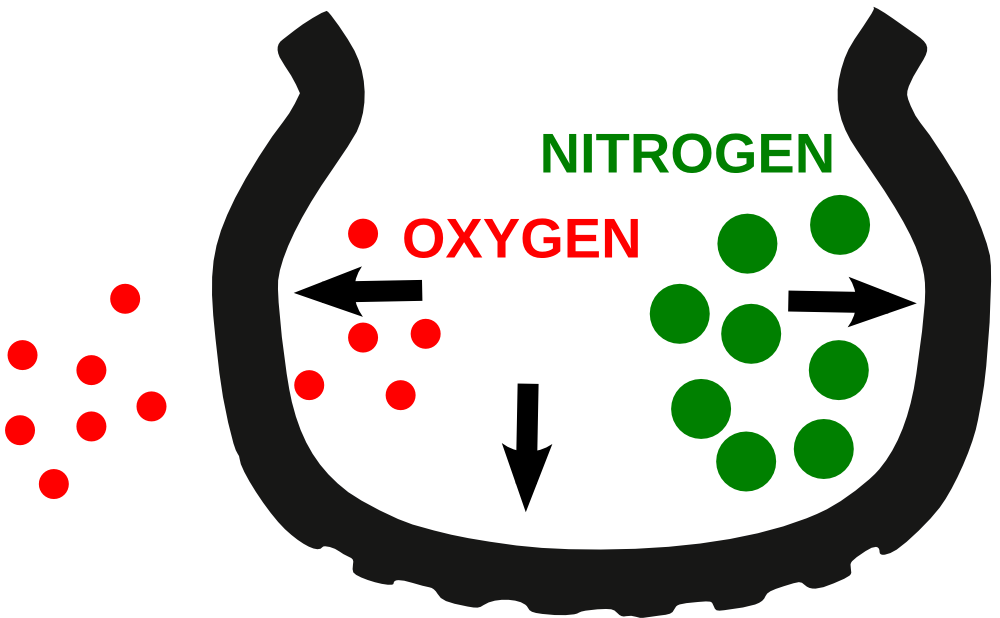
Nitrogen is a slow inactive gas. This means its much tougher for nitrogen molecules to escape the tiny spaces between rubber molecules thus maintaining tire pressure for a longer time. Oxygen is a fast active gas so its easier for air to escape the tire when it has higher oxygen levels.
Nitrogen is a dry gas. Auto racers began to use nitrogen to better control tire pressures. Their tires can get very hot small pressure changes can have a large impact on handling and they often run much lower pressures than we do on the street.
Most of us cant feel a 3 psi tire pressure change in our cages and 1 psi in our bikes on the street. Nitrogen particles are larger than oxygen and this makes it more difficult for nitrogen to leak out of your tires. This stability in pressure makes it ideal for race tracks where the temperature of the tires usually changes and eventually heats up during a race.
Stable pressure is equally important in your everyday car. However the presence of air in your tires instead of nitrogen can easily jeopardize this pressure.