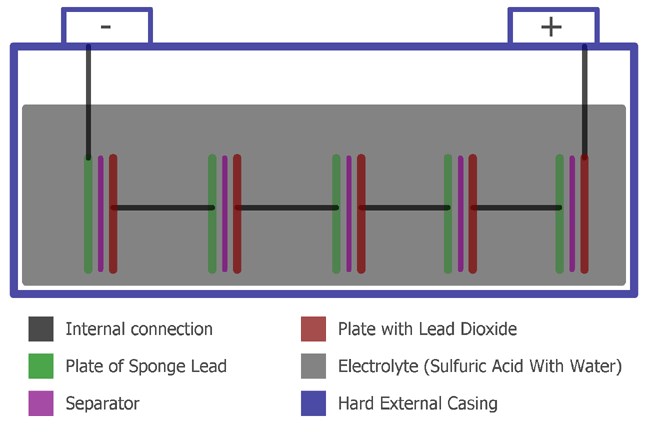
Lead-acid accumulator - a battery with lead electrodes with dilute sulphuric acid as the electrolyte. These two plates are separated using a separator which is an insulating material.

This makes it ideal for many applications.
Lead acid accumulator battery. Chemical additives have been used ever since the leadacid battery became a commercial item to reduce lead sulfate build up on plates and improve battery condition when added to the electrolyte of a vented leadacid battery. Such treatments are rarely if ever effective. Two compounds used for such purposes are Epsom salts and EDTA.
Epsom salts reduces the internal resistance in a weak or damaged battery and may allow a small amount of extended life. The lead acid batteries used in cars have undergone significant changes with time thus paralleling the car evolution particularly in terms of electric and electronic equipment. Until mid 70s the accumulators were basically formed by.
Positive and negative grids with PbSb alloys 4-6 Sb. The most common type of heavy duty rechargeable cell is the familiar lead-acid accumulator car battery found in most combustion-engined vehicles. A simple lead-acid cell consisting of strips of lead and an electrolyte of dilute sulfuric acid is constructed and charged for different lengths of time.
A SIMPLE explanation for how a Lead Acid Battery works. This tutorial covers the working principle of a Lead Acid Battery and how it is constructed. The lead acid battery is very well established.
It has been in use for over 150 years and is currently one of the mainstays of the automotive industry. The lead acid battery has a high current capability low cost and it is tolerance to abuse. This makes it ideal for many applications.
Lead Acid Battery Definition. The battery which uses sponge lead and lead peroxide for the conversion of the chemical energy into electrical power such type of battery is called a lead acid battery. The lead acid battery is most commonly used in the power stations and substations because it has higher cell voltage and lower cost.
A Lead Acid Battery consists of Plates Separator and Electrolyte Hard Plastic with a hard rubber case. In the batteries the plates are of two types positive and negative. The positive one consists of Lead dioxide and negative one consists of Sponge Lead.
These two plates are separated using a separator which is an insulating material. Materials used are led peroxide PbO 2 sponge led Pb and dilute sulfuric acid H 2 SO 4 Positive plate of lead acid battery is made of PbO 2 which is dark brown hard and brittle substance. Negative plate of lead acid battery is made of pure led in soft sponge condition Pb.
Here H 2 SO 4 uses water to acid ratio of 31. When you connect it to the battery an it should draw 100-200mA. Set your psu to 14v or connect your charger and let it sit for day or two.
After that your battery should draw 500-1000mA. Then just leave battery and calculate how long battery should charge. Suitable for 1-1000AH 12V Lead-Acid Battery charger.
The DD30CRTA is a PWM switch-mode battery charger controller for 12V lead-acid battery in a small package using few external components. The DD30CRTA is specially designed for charging 12V lead-acid battery with trickle charge constant current charge over-charge and float charge mode. This is because the self-discharge rate of an SLA battery is 5 times or greater than that of a lithium battery.
In fact many customers will maintain a lead acid battery in storage with a trickle charger to continuously keep the battery at 100 so that the battery life does not decrease due to storage. The lead acid battery uses the constant current constant voltage CCCV charge method. A regulated current raises the terminal voltage until the upper charge voltage limit is reached at which point the current drops due to saturation.
The charge time is 1216 hours and up to 3648 hours for large stationary batteries. Buy Battery Charger Module Lead-Acid Battery 3A 12V 1-1000AH Accumulator Lead Acid Battery Cell Storage Battery Charger ModuleWithout Terminal. Lead-acid accumulator - a battery with lead electrodes with dilute sulphuric acid as the electrolyte.
Each cell generates about 2 volts lead-acid. Lead-acid accumulator - definition of lead-acid accumulator by The Free Dictionary. Chemical Action of Lead Acid Battery The battery cells can be recharged by reversing the direction of discharge current in the battery.
This is done by connecting positive terminal of a DC source with positive terminal of the battery and similarly negative terminal of the DC source with negative terminal of the battery. China Heli Forklift Accumulator Lithium Battery for Sale Find details about China Forklift Battery Forklift Battery for Sale from Heli Forklift Accumulator Lithium Battery for Sale - Anhui Leading Forklift Parts Co Ltd. Here is how it works.
When the lead acid battery accepts charge the sulfuric acid gets heavier causing the specific gravity SG to increase. As the SoC decreases through discharge the sulfuric acid removes itself from the electrolyte and binds to the plate forming lead sulfate. A lead-acid battery is the most inexpensive battery and is widely used for commercial purposes.
It consists of a number of lead-acid cells connected in series parallel or series-parallel combination. A lead-acid cell basically contains two plates immersed in electrolyte dilute sulphuric acid ie. H 2 SO 4 of specific gravity about 128.
Best Trolling Motor Battery Reviews. Some novice anglers might imagine that theyll power their angling motor with a normal SLI-type lead-acid accumulator. The reality is its not ideal to use a lead-acid accumulator.
Mistreatment automotive batteries on a angling motor can solely destroy the motor and therefore the battery. Automotive batteries can not be recharged and be brought down on.